Mechanisms of Action Against Pain Signal Propagation, Gastrointestinal Inflammation, and its Role in Neurogenesis and Neuroprotection
This is a comprehensive review of existing scientific work on Cannabichromene and its mechanisms of action inside the human Endocannabinoid cell signaling system. As it is shown, CBC can inhibit pain signal propagation in the body, inhibit inflammatory response in the immune system, and act as a neuroprotector of the central nervous system cells. The non-psychoactive cannabinoids CBC and CBD are known to modulate the activity of proteins involved in nociceptive mechanisms, including transient receptor potential (TRP) channels of vanilloid type-1 (TRPV-1) and of ankyrin type-1 (TRPA-1), as well as proteins facilitating endocannabinoids inactivation. In this paper CBC Phyto-Cannabinoid and the Endocannabinoid system are discussed, as well as the mechanisms of action of CBC are presented, along with compelling evidence of their action against pain signal propagation, inflammation, and neuroprotection.
Phyto-Cannabinoids and Endocannabinoid Signaling System
Cannabichromene (CBC) in it’s acid form (CBCA) is one of the major Phyto- cannabinoids that occurs in the cannabis plant as a product of synthase reactions between the Mother Cannabinoid (CBGA) and various enzymes that exist in the particular strain of cannabis in the environment that provides the necessary thermal, photolytic and oxidative conditions. Along with CBCA, the synthase reactions also give birth to the well-known Cannabidiolic Acid (CBDA) and Tetrahydrocannabidiolic Acid (THCA) major Phyto-cannabinoids(2). After the cannabis plants are harvested and decarboxylated, the acid forms of these 3 major Phyto-cannabinoids turn into their base forms (CBC, CBD, and Δ9-THC), which are considered more active than their acid forms (Figure 1).
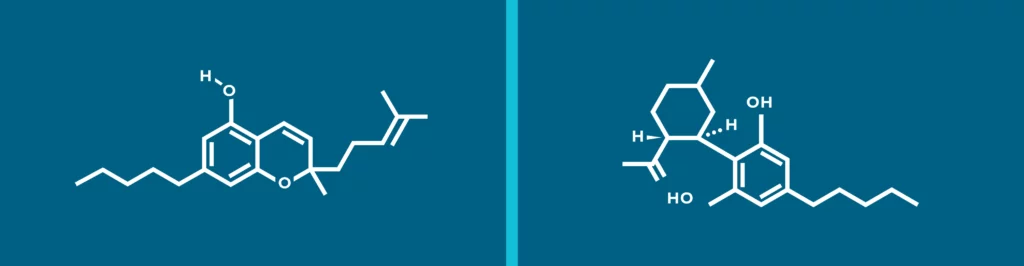
Figure 1. Chemical structure of CBC (left) and CBD (right).
In the presence of pain and inflammation stimuli in the body cells of the central nervous system biosynthesize endocannabinoids anandamide (AEA) and 2-arachydonoylglycerol (2-AG), which are lipid mediators synthesized ‘on demand’ from cell membrane phospholipids(3). Once synthesized, endocannabinoids, activate cannabinoid CB1 and CB2 receptors to elicit a biological response (see Figure2), after which they are inactivated through re-uptake (facilitated by the putative endocannabinoid membrane transporter (EMT)] and enzymatic degradation by fatty acid amide hydrolase (FAAH), and MAGL “killer” enzymes(4,5). The CB1 And CB2 cannabinoid receptors are part of the endocannabinoid signaling system which also includes the enzymes that synthesize and degrade endocannabinoids, as well as possible transporters. The molecular mechanisms for regulating lipid-based signaling events such as cannabinoid receptor signaling are not yet completely understood, although significant progress has been made(6,7). Activation of CB1 and CB2 receptors results in some natural pain and inflammatory stimulus suppression(8), but the amplitude and duration of that suppression is regulated by how fast they are broken down, so this anti-nociceptive effect is weak and short-lived (only about 4-5 minutes in vivo)(9).
Preventing Endocannabinoid Inactivation
As soon as the Endocannabinoids are inactivated and broken down by the “killer” enzymes, CB1 and CB2 receptors are inactivated and the pian and inflammation relief stops. To prolong the pain relief the Endocannabinoid inactivation must be inhibited. Examining the mechanism of action of the Cannabichromene (CBC) we see that it bonds to TRPV-1 cell channels and desensitizes them, which leads to inhibition of the “killer” enzyme release.
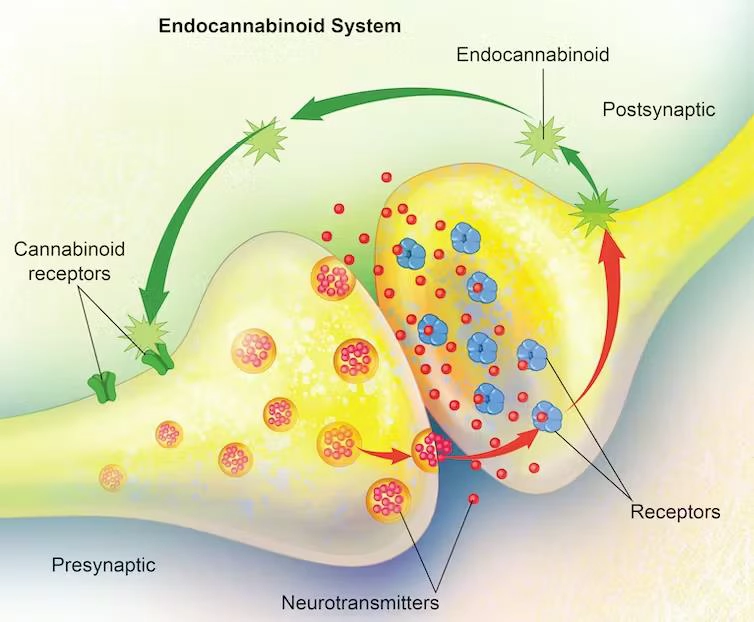
Figure 2. The endocannabinoid system – consisting of the endocannabinoids and the cannabinoid receptors – regulates nerve cell communication at the synapse, thereby playing a role in a variety of bodily functions. Carolina Hrejsa, CMI/iStock/Getty Images Plus via Getty Images.
This leads to CB1 and CB2 receptors remaining active for a much longer time, and a longer pain relief(1). This also leads to Endocannabinoid accumulation in the area where they are released. In time, more and more CB1 and CB2 receptors get activated, which leads to more pain relief. However, at some point the concentration of Endocannabinoids reaches a point where they activate the TRPA-1 channel, which is a pain signal releasing channel, which means that further accumulation of Endocannabinoids in the system is counter-productive to the pain relief. Now, looking at the mechanism of action of Cannabidiol (CBD) we see that CBD bonds to the TRPA-1 channels and desensitizes them, which means that if CBD and CBC are taken together, CBC will desensitize the TRPV-1 channel, which will inhibit the “killer” enzyme release, and CBD will inhibit the TRPA-1 channel, which will inhibit propagation of pain signals as a result of Endocannabinoid concentration in the area. As a result, the pain relief is better and longer. This can be seen in the animal trial performed by (Sabatino et. al.) that measured the electrical activity of neurons as well as tail flick latencies to thermal stimuli of rats that were injected with CBC, CBD and saw that CBC and CBD dose-dependently reduce the on-going activity of ON and OFF pain neurons, inhibited endocannabinoid inactivation, and reduced the pain response to the tail-flick test(1).
In the study by (Sabatino Maione et.al) 260 Wistar rats were divided by groups of 12-16 animals per treatment. Group-1 received an intra-vl-PAG injection of 200nL of vehicle (0.2% dimethyl sulfoxide, DMSO, in artificial CSF). Group-2 received microinjections of (1.5, 3 and 6 nmol) of CBD in the same vehicle. Group-3 received microinjections of (3 and 6 nmol) of CBC in the same vehicle. There were other groups that received injections of various synthetic TRPV-1 and TRPA-1 channel and CB1 and CB2 receptor antagonists along with the cannabinoids to see how the study parameters change in each case (see the study by Sabatino Maione et.al)(1). but we will only discuss the vehicle, CBC and CBD by themselves, and then draw conclusions about the mechanism of action of the CBC/CBD combination.
Designations
Number of rat tail withdrawal reflexes per 10 seconds was called “Spikes (s-1)”. Latency time to the tail withdrawal reflex was designated as “Tail Flick Latency (s)”.
Results of the (Sabatino Maione et.al) Trial
Sabatino Maione et.al. found that CBD at 3nmol concentration in the vehicle significantly elevated the concentration of 2-AG Endocannabinoid by 2.6-fold, but not AEA Endocannabinoid levels. CBC at 6nmol concentration in the vehicle significantly elevated both 2-AG levels, by almost 3.9-fold and AEA levels, by almost 1.7-fold)(1) (see Table 1)
When examining the mechanism of action of Cannabidiol (CBD), it’s observed that CBD binds to and desensitizes the TRPA-1 channels. This desensitization means that when CBD and Cannabichromene (CBC) are used concurrently, there are complementary effects.
Specifically, CBC desensitizes the TRPV-1 channel, inhibiting the release of “killer” enzymes. Concurrently, CBD inhibits the TRPA-1 channel. This dual action effectively hampers the propagation of pain signals due to the concentration of endocannabinoids in the area, leading to enhanced and prolonged pain relief.
Evidence of Animal Studies
Evidence of this synergistic effect was noted in an animal study conducted by Sabatino et al. This study, which measured the electrical activity of neurons and tail flick responses to thermal stimuli in rats injected with CBC and CBD, found that the combination of these compounds dose-dependently reduced the activity of both ON and OFF pain neurons. Additionally, it inhibited endocannabinoid inactivation, and diminished the pain response in the tail-flick test(1).
| Compound | AEA (pmol/g tissue) | 2-AG (nmol/g tissue) |
| Vehicle | 90.1 ± 15.8 | 7.8 ± 2.1 |
| CBD (3 nmol) | 107.7 ± 14.4 | 20.5 ± 4.4 |
| CBC (6 nmol) | 148.7 ± 21.0 | 30.1 ± 6.1 |
This is the experimental proof that CBC inhibits downregulation of Endocannabinoid, which results in significant increase in Endocannabinoid concentration, but how does that relate to the anti-nociception?
They also found that CBD and CBC decrease the number of Spikes per 10 second interval from 7-8 for the vehicle (placebo) to about 2-4 in a dose dependent manner, which indicates a decrease in pain signals. Maximum decrease of number of spikes was observed when 3nmol of CBD was injected, and 6nmol of CBC was injected. Figure 3 shows the effect of Vehicle, CBD(1.5, 3, and 6 nmol) and CBC (3 and 6 nmol) on the Tail-Flick latency.
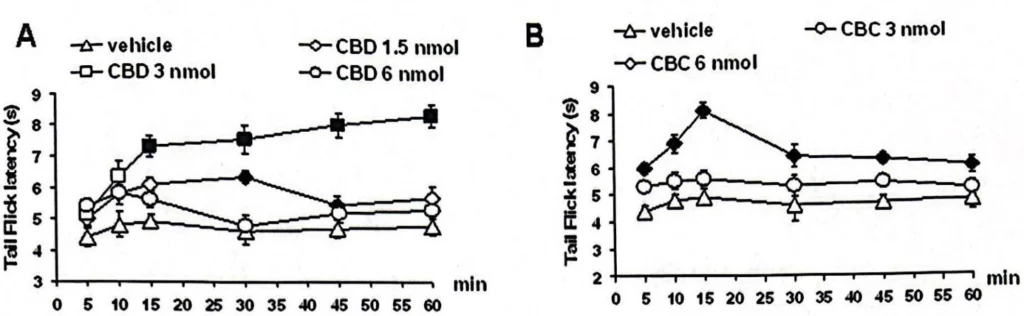
Figure 3. “A” shows the effect of the vehicle and CBD (1.5, 3 and 6 nmol), “B” shows the effect of vehicle and CBC (3 and 6 nmol) on the tail-flick latency at different concentrations of CBD and CBC)(1).
Discussion
The (Sabatibo et.al) trial(1). proves that CBC at 6nmol most effectively activates TRPV-1 channel and desensitizes it, resulting in inhibition of Endocannabinoid downregulation, by preventing a release of “killer” enzymes that break down Endocannabinoids. This leads to Endocannabinoid concentration and most effective anti-nociceptive activity by the body itself (without synthetic analgesic medication). But that anti-nociceptive effect doesn’t last longer than a few minutes, because Endocannabinoids activate the TRPA-1 pain signal channel which increases the pain, which can be see in Figure 2 (B) when the tail-flick latency increases from about 6 seconds to over 8 seconds in 15 minutes and then decreases back to about 6 seconds over the next 15 minutes, whereas CBD at 3nmol increases the tail-flick latency to about 7 seconds in 15 minutes, and keeps increasing it to about 8 seconds in the next 45 minutes and on.
This means if we administer CBD(3nmol) and CBD(6nmol) together in the vehicle, the tail-flick latency would be as in the Figure 4 below:
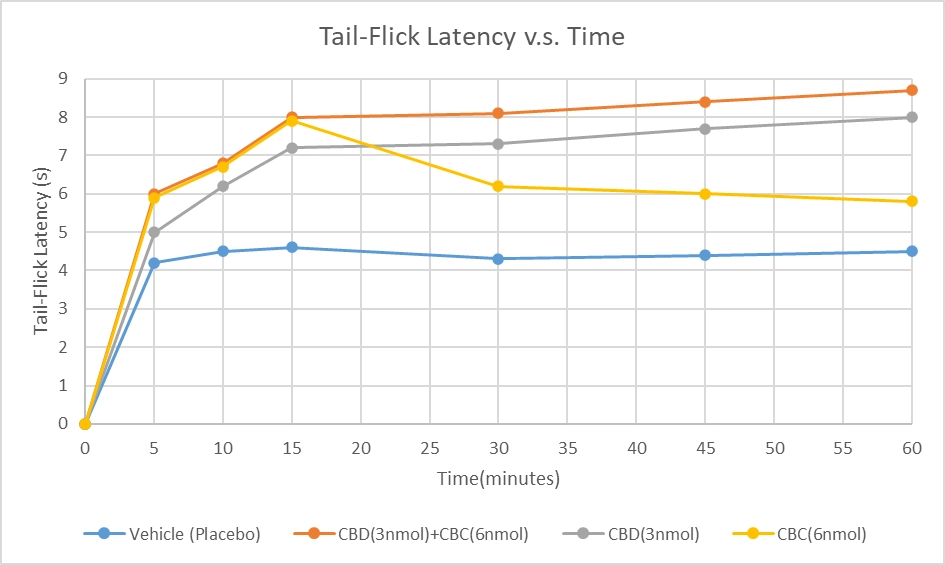
Figure 4. Comparison between Tail-Flick Latency between the Vehicle, CBD(3nmol), CBC(6nmol), and a theoretical representation of what CBD(3nmol) and CBC(6nmol) would result in if they were taken together.
This shows that if CBD(3nmol) and CBC(6nmol) are taken together, the anti-nociceptive effects are felt much quicker and stronger than the vehicle, or if they were taken separately. The duration of the anti-nociceptive effect is also much longer and may show increase over time.
Anti-inflammatory Effect of CBD
Now that we have established the anti-nociceptive effect of Cannabichromene, as well as reviewed the mechanism of its action on inhibition of Endocannabinoid down-regulation by means of desensitizing the TRPV-1 and TRPA-1 channels alone and in combination with Cannabidiol, we switch our attention on the Anti-Inflammatory action of CBC. Endocannabinoids and TRPA-1 channel modulate gastrointestinal motility, and the effect of CBC on the GI motility of mice was investigated in the (Izzo, et. al.) study, where inflammation was induced in the mouse small intestine by croton oil. Endocannabinoid (anandamide and 2-arachidonoyl glycerol), palmitoylethanolamide and oleoylethanolamide levels were measured by LC-MS; TRPA1 and cannabinoid receptors were analyzed by quantitative RT-PCR(13).
The results showed that Croton oil administration was associated with decreased levels of Anandamide(AEA) and Palmitoyl Ethanolamide Endocannabinoids, up-regulation of TRPA1 and CB1 receptors and down-regulation of CB2 receptors, which causes an inflammatory response, but CBC normalized croton oil-induced hypermotility and reduced preferentially EFS-versus ACh-induced contractions, hence reducing the GI tract inflammation. In other words, CBC selectively reduces inflammation-induced hypermotility in-vivo in a manner that is not dependent on cannabinoid receptors or TRPA1(13). Figure 5 represents the electrically-induced contractions in the mouse isolated ileum of control (A) and croton oil-treated mice(B): inhibitory effect of CBC (10-8–10-4 M) alone (vehicle) or in the presence of w-conotoxin, which causes more inflammation (10-8 M). Each point represents mean ± SEM of 7–8 experiments(13). Both in control mice (A) and in croton oil-treated mice (B), w-conotoxin reduced the inhibitory effect of CBC, but the inflammation inhibition is still significant in-spite of the inflammatory factors that exist.
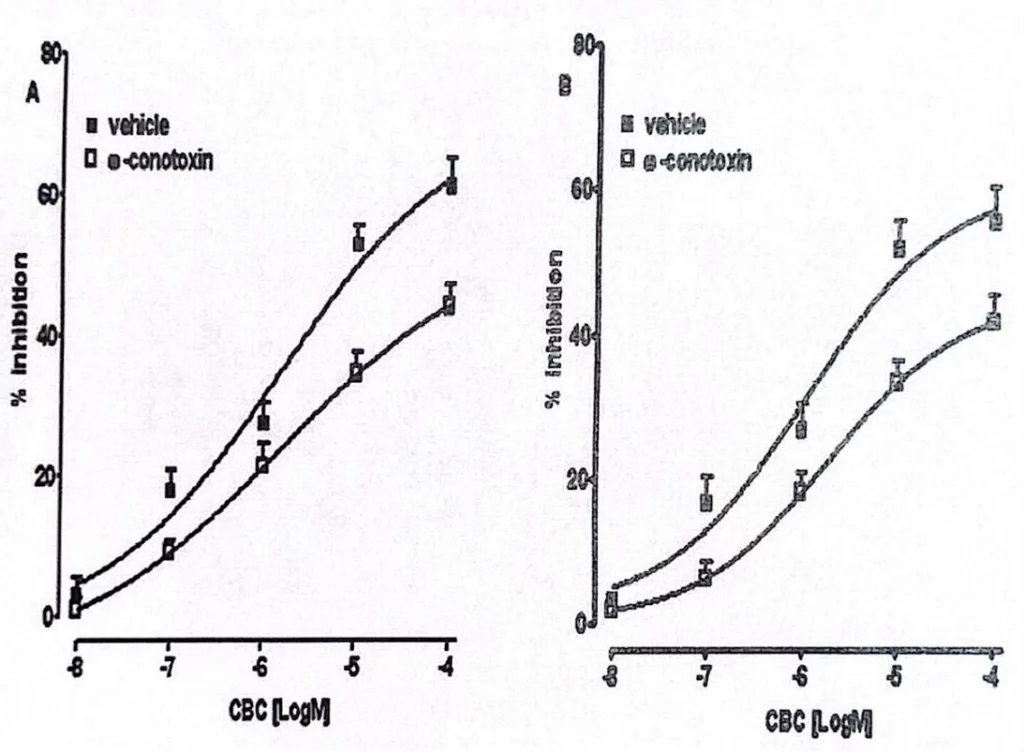
Figure 5. Electrically-induced contractions in the mouse isolated ileum of (A) control and (B) croton oil-treated mice(13).
Discussions
Little is known about the effects of non-psychotropic Phyto-cannabinoids in the gut. While cannabidiol, the most studied among the non-psychotropic Phyto- cannabinoids, was shown to exert a protective effect in the inflamed gut(14, 15, 16, 17), there are very little data in existence in the literature concerning the effect of CBC in the digestive tract. In the (Izzo, et. al.) study(13), it was proven that CBC did not affect upper gastrointestinal transit, colonic propulsion or whole gut transit in healthy mice in vivo, however it reduced croton-oil induced intestinal inflammation and the results clearly show that CBC is pharmacologically active in vivo only when intestinal homoeostasis is perturbed by an inflammatory stimulus.
The observation that CBC administration is not associated with constipation under physiological conditions is relevant as one of the major side effects associated with opiate administration (the most known agent able to reduce intestinal motility) is constipation(13,18). Interestingly, it was also proven that CBC mechanism of inflammation reduction did not involve TRPA-1, TRPV-1, or CB1 and CB2 receptors at all. Instead there is evidence that CBC inhibits inflammation, at least in part, by limiting the availability of intraneuronal Ca2+ via inhibition of N-type Ca2+ channels(19). Although the precise mechanism of the inhibitory effect of CBC requires further studies, the present results are of potential clinical interest because intestinal dysmotility in inflammatory diseases is a well-recognized and clinically accepted phenomenon(20), in which the only drugs currently available to counteract it are often associated with constipation(18).
Role of CBC in Neurogenesis and Neuroprotection.
Neurogenesis is the process by which new neurons are formed in the brain. Neurogenesis is crucial when an embryo is developing, but also continues in certain brain regions after birth and throughout our lifespan(11) (see Figure 6). The mature brain has many specialized areas of function, and neurons that differ in structure and connections. The hippocampus, for example, which is a brain region that plays an important role in memory and spatial navigation, alone has at least 27 different types of neurons(11). The incredible diversity of neurons in the brain results from regulated neurogenesis during embryonic development. During the process, neural stem cells differentiate—that is, they become any one of a number of specialized cell types—at specific times and regions in the brain(11) (see Figure 7).
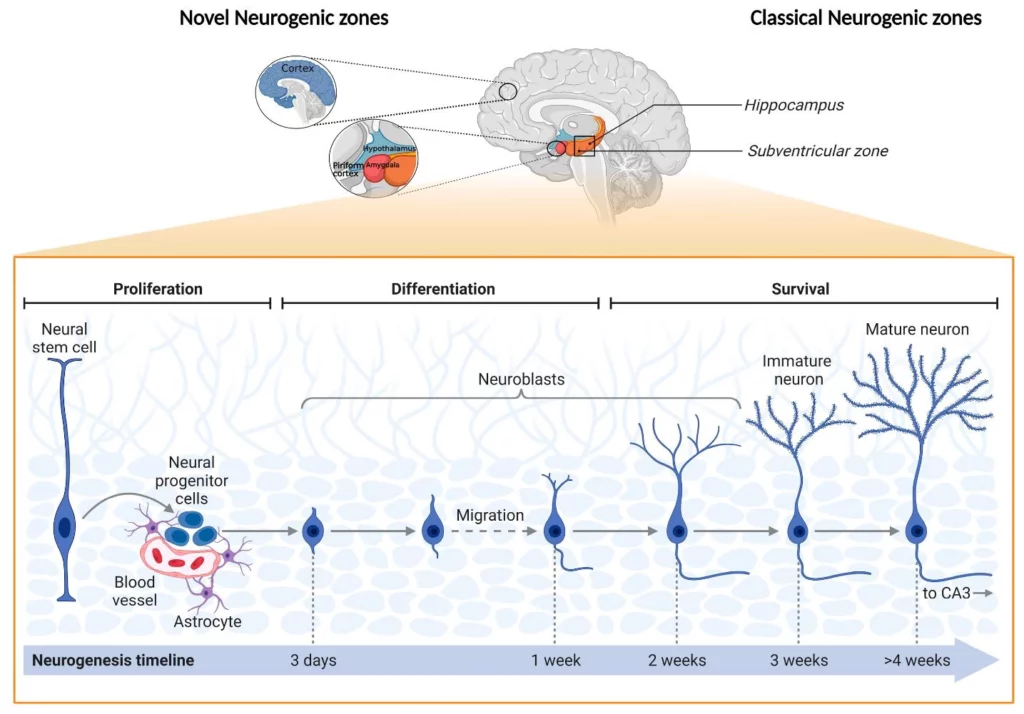
Figure 6. Schematic representation of “novel” and classical adult neurogenic zones, as well as their main cellular stages (12).
Stem cells can divide indefinitely to produce more stem cells, or differentiate to give rise to more specialized cells, such as neural progenitor cells. These progenitor cells themselves differentiate into specific types of neurons(11).
The neural stem/progenitor cell (NSPC) population in the adult brain is essential for brain plasticity under normal physiological conditions as well as during the recovery from brain injuries(10).
To test the effect of CBC on adult neurogenesis directly through the action on NSPCs, (Noriko Shinjyo, Vincenzo Di Marzo) tested the effects of these compounds on the viability of cultured NSPCs derived from the adult mouse brain, using the MTT assay and found that CBC significantly raised the cell viability(10), proving Neuroprotective properties as well as its significant role in Neurogenesis.
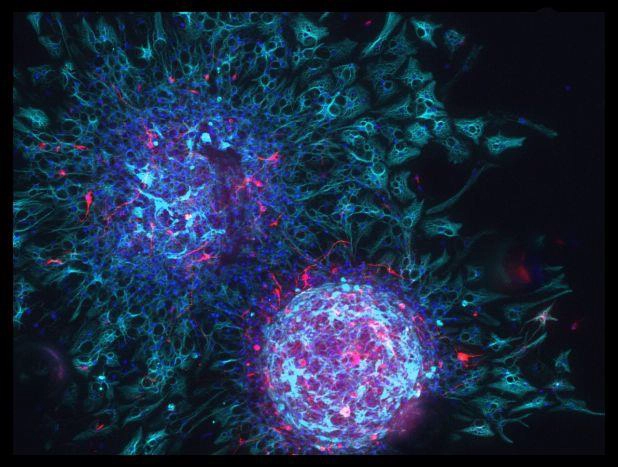
Figure 7. Neural stem cells can produce new neural cells of any type. When stem cells from the brain are isolated and grown in a dish, they continuously divide and create large spherical masses of cells, similar to the two shown here. Each spherical mass, called a neurosphere, is produced by a single stem cell. When exposed to different chemicals, the cells turn into either neurons (red) or glia (cyan). Cell nuclei are shown in dark blue. (Image: Chanel Taylor / QBI) (11)
Conclusion
The CBC studies are limited and mostly conducted on animals like mice, but it does show promise and necessity for more research in this space, particularly human clinical studies. As was shown in this review, CBC only goes to work if there are issues with pain, inflammation in the gastrointestinal tract, or neurogenesis, or neuroprotection. If there is significant pain it bonds to TRP cell channels and blocks production of “killer” enzymes, which in turn inhibits Endocannabinoid breakdown and prolongs pain relief initiated by the CB receptors, especially if taken with CBD. If there is GI inflammation it inhibits the N-type Ca2+ channels in GI cells and inhibits the inflammatory response of contracting and expanding GI tract. If there are issues with cell viability in the brain it acts as a neuroprotector.
Sources
- Sabatino Maione, Fabiana Piscitelli, Luisa Gatta, Daniela Vita, et. al. “Non-psychoactive cannabinoids modulate the descending pathway of antinociception in anesthetized rats through several mechanisms of action”. British Journal of Pharmacology, DOI: 10.1111/j.1476-5381.2010.01063.x
- Brian F. Thomas, Mahmoud A. ElSohly“The Botany of Cannabis sativa L.”. The Analytical Chemistry of Cannabis, (Quality Assessment, Assurance, and Regulation of Medicinal Marijuana and Cannabinoid Preparations) 2016, Pages 1-26.
- Angelo A Izzo, Raffaele Capasso, Gabriella Aviello, Francesca Borrelli. “Inhibitory effect of cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice” British Journal of Pharmacology, 2012 Jun; 166(4): 1444–1460
- Di Marzo V.“Targeting the endocannabinoid system: to enhance or reduce?” Nature Review Drug Discovery. 2008;7:438–455
- Pertwee RG. “Emerging strategies for exploiting cannabinoid receptor agonists as medicines”. British Journal of Pharmacology 2009;156:397–411
- Patricia H. Reggio“Endocannabinoid Binding to the Cannabinoid Receptors: What Is Known and What Remains Unknown”. Curr Med Chem. 2010; 17(14): 1468–1486
- Katona L, Freund TF“Endocannabinoid signaling as a synaptic circuit breaker in neurological disease”. Nat. Med. 2008;14:923–930
- Adarsh Thomas Anthony, Shermeen Rahmat, Prerna Sangle“Cannabinoid Receptors and Their Relationship With Chronic Pain: A Narrative Review”, Cureus. 2020 Sep; 12(9): e10436
- Jason Socrates Bardi“Turning Off Pain’s Pathways”, The Scripps Research Institute Journal, Volume 1, Issue 22, August 13, 2001
- Noriko Shinjyo, Vincenzo Di Marzo“The effect of cannabichromene on adult neural stem/progenitor cells” Neurochemistry International 63 (2013) 432-437
- “What is Neurogenesis?” The University of Queensland, Australia, Queensland Brain Institute
- Andrais Vaz, Ines Ribeiro, Luisa Pinto “Frontiers in Neurogenesis”, Cells 2022, 11(22), 3567
- Angelo A Izzo et. al.“Inhibitory effect of Cannabichromene, a major non-psychotropic cannabinoid extracted from Cannabis sativa, on inflammation-induced hypermotility in mice. Br J Pharmacol 2012 Jun;166(4):1444-60. doi: 10.1111/j.1476-5381.2012.01879.x
- Capasso R, Borrelli F, Aviello G, Romano B, Scalisi C, Capasso F et al. (2008a) “Cannabidiol, extracted from Cannabis sativa, selectively inhibits inflammatory hypermotility in mice. British Journal of Pharmacology, 154: 1001–1008
- Borrelli F, Izzo AA (2009)“Role of acylethanolamides in the gastrointestinal tract with special reference to food intake and energy balance.” Best Pract Res Clin Endocrinol Metab 23: 33–49
- Jamontt JM, Molleman A, Pertwee RG, Parsons ME (2010).“The effects of Delta-tetrahydrocannabinol and cannabidiol alone and in combination on damage, inflammation and in vitro motility disturbances in rat colitis.” British Journal of Pharmacology, 160: 712–723
- Alhamoruni A, Wright KL, Larvin M, O’Sullivan SE (2012).“Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability.” British Journal of Pharmacology, 165: 2598–2610
- Jafri S, Pasricha PJ (2001).“Agents used for diarrhoea, constipation, and inflammatory bowel disease; agents used for biliary and pancreatic disease.” The Pharmacological Basis of Therapeutics, 10th edn. McGraw-Hill: New York, pp. 1037–1058.
- Leal-Cardoso JH, Lahlou S, Coelho-de-Souza AN, Criddle DN, Pinto Duarte GI, Santos MA et al. (2002).“Inhibitory actions of eugenol on rat isolated ileum.” Can J Physiol Pharmacol 80:901–906
- Ohama T, Hori M, Ozaki H (2007).“Mechanism of abnormal intestinal motility in inflammatory bowel disease: how smooth muscle contraction is reduced?” Journal of Smooth Muscle Res 43: 43–54

Reviewed By:
Vardan Ter-Antonyan
Manager, R&D Formulations
Research & Development



